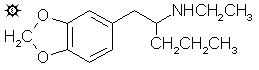
#78 ETHYL-K
2-ETHYLAMINO-1-(3,4-METHYLENEDIOXYPHENYL)PENTANE; N-ETHYL-1-(1,3-BENZODIOXOL-5-YL)-2-PENTYLAMINE
SYNTHESIS: A solution of 120 mg mercuric chloride in 160 mL H2O was
poured over 4.7 g aluminum foil (Reynolds Wrap, regular weight, cut
into 1 inch squares) and allowed to stand until the amalgamation was
well underway (about 30 min). The H2O was then drained and the foil
washed with 2x200 mL H2O with thorough draining. There was then
added, in sequence and with good swirling and agitation between each
addition, 8.5 g ethylamine hydrochloride dissolved in 7 mL H2O, 21 mL
IPA, 17 mL 25% NaOH, 7.1 g 1-(3,4-methylenedioxyphenyl)-2-pentanone
(see the recipe for METHYL-K for its preparation), and finally 40 mL
IPA. The reaction mixture was periodically heated on the steam bath
to keep the reaction moving and active. After all the metal had been
consumed, the mixture was filtered, and the filter cake washed with
MeOH. The solvent was removed from the combined filtrate and
washings, and the residue suspended in 800 mL dilute HCl. This was
washed with 3x100 mL Et2O, made basic with 25% NaOH, and extracted
with 3x100 mL CH2Cl2. The pooled extracts were stripped of solvent
under vacuum yielding a residue of 6.3 g of an amber oil. This was
distilled at 115-125 °C at 0.4 mm/Hg to give 5.61 g of an almost white
liquid which was dissolved in 28 mL IPA, neutralized with concentrated
HCl, and diluted with 100 mL anhydrous Et2O. The resulting clear
solution became cloudy, then set up in a cottage cheese texture, and
then all broke up to a beautiful loose solid. This was filtered, Et2O
washed and air dried to give 5.99 g
2-ethylamino-1-(3,4-methylenedioxyphenyl)pentane hydrochloride
(ETHYL-K) with a mp of 157-158 °C. Anal. (C14H22ClNO2) C,H.
DOSAGE: (greater than 40 mg).
DURATION: unknown.
QUALITATIVE COMMENTS: (with 40 mg) There was a paresthetic twinge in
my shoulder area at about an hour--other than that, absolutely
nothing.
EXTENSIONS AND COMMENTARY: And that is as high a dose as has
apparently ever been tried with ETHYL-K. The compounds with the
hexane chain (L-series) rather than the pentane chain of the K-series
have been made, but they have been spun into the recipe for METHYL-K.